![[Translate to en-CH:]](/fileadmin/_processed_/e/3/csm_BBV_Motiv_2015_hires_e5d41fc08b.jpg)
![[Translate to en-CH:]](/fileadmin/_processed_/a/2/csm_BBV_6_REV1835_232a666f44.jpg)
Cathodic corrosion protection
Cathodic corrosion protection of steel in concrete structures

Cathodic corrosion protection (CP) is new technology that imparts currents through the concrete structure in an intelligent and protective way to either minimise the onset or reduce corrosion problems affecting the structure.
Cathodic corrosion has been proven in many countries to be an effective method to prevent or avoid the loss of reinforcement in concrete due to corrosion (many millions m2)
The application of inducing a protective current in the concrete structure has been continuously developed and improved over many years. Over this time the available systems have evolved through improved planning assessment, improved materials, better installation techniques and the incorporation of continuous monitoring systems. Implementation of CP systems in concrete structures are now covered under specification DIN ISO EN 12696: 2012
The conventional method of repairing concrete structures containing reinforcement corrosion generally involves the removal of the cracked or palled concrete areas. This is followed by the subsequent reinstatement of the corroded reinforcement and concrete substrate. In cases with minor isolated or localized corrosion – this approach is still suitable.
Major disadvantages to the conventional repair methods include:
- large portions of otherwise healthy concrete must be removed in order to reinstate the reinforcement,
- Bonding between the reinstated concrete and the existing structure, which could affect the long term durability of the repair.
The installation of a CP system offers advantages
- reduction in the amount of concrete to be removed and reinstated
- Recording, quantifying and monitoring the structural state of the building via built-in sensors.
After the application of this system the state of the building is more or less "frozen", and the remaining service life of the structure is extended as long as the protective current is maintained within the structure.
Corrosion
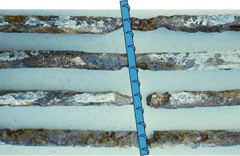
The initial signs of corrosion of reinforcement in concrete structures, are usually minor cracks accompanied with rust stains on the concrete surface. Subsequently, cracks propagate in the structure leading to concrete spalling. (By-products of corrosion have a volume 5-7 times larger than the steel in the original state).
The further the concrete weakens the wider the cracking occur. As this cycle of deterioration occurs there is higher level of exposure and infiltration of foreign materials (water and air) within the concrete, which compounds by further accelerating the rate of chemical reaction (corrosion). In extreme cases, the loss of steel cross-section leads to a reduction in the tensile strength and subsequent failures to the structural capacity of the structure.
Reinforcement which is totally covered with concrete (i.e. absence of any foreign material) will not corrode due to its passivity, and the presence of a protective thin iron oxide layer. If this state between the steel and concrete can be maintained, the rate of corrosion can be considered negligible
The passivity of the steel can be changed by two mechanisms (in the presence of moisture and oxygen):
- Influence by carbon dioxide – this process involves the reduction of the pH value and leads to a uniform, extensive loss of passivation (= corrosion induced by carbonation)
- Presence of chloride ions, which locally damage the passive oxidizing film and cause pitting corrosion (= chloride-induced corrosion e.g. de-icing salts, accelerators, etc.)
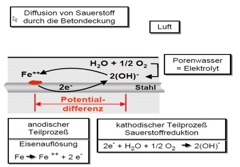
All types of corrosion are accompanied by an induced current flow in a structure, and involves the flow of current away from the metal into an adjacent Electrolyte. As a result of this discharge of current, metal ions are released from the metal component and begin to corrode.
Cathodic corrosion protection
Cathodic protection is an electro-chemical protection process in which a direct electrical current is induced through the Electrolyte (pore water solution in the concrete) to provide a protection to the tensile reinforcement. The result of this current is to induce a cathodic polarization on the perimeter surface of the reinforcement, i.e. there is a shift in the electrochemical potential "E" of the steel towards more negative values. This prevents metal ions from being released from the metal surface (see corrosion). The anodic sub-process (iron dissolution) of corrosion is suppressed or prevented (metal removal rate <0.01 mm / A). The result is a change in potential to values at which the entire steel becomes cathodic compared to the anode, hence the term “cathodic protection”.
A CP system creates an electric field between the reinforcement and the anode, which in addition to the change in potential leads to two further positive side effects in the immediate vicinity of the steel.
- Under the influence of the modified flow of current, all negatively charged ions (OH-, CL-, SO42-) migrate away from the reinforcement, towards the Anode. The effect of the electro-migration in the normal CP operation, ensures a separation between the chloride ions and the steel surface, which significantly reduces the chance of further risk of corrosion.
- As a result of the induced current for the CP system, a thin ‘carbonated’ layer is induced around the reinforcing steel resulting in an elevated pH value. As such the steel is changed to a more ‘passive’ state, meaning that corrosion can no longer be triggered, or able to progress. This effect can be observed at low chloride concentrations.
The application of a CP-B system in a structure involves the introduction of new Anode(s). The Anode(s) are either drilled, slotted or mounted on the surface of the concrete in a pre-defined arrangement. After mounting of Anode(s) they are connected to the positive side of a new electrical source, whilst the concrete reinforcement is secured to the negative side of the electrical source, and hence inducing the required flow of current within the structure. Various checks are required on the systems prior to connection of the Anode, primarily the continuity/connectivity of the existing reinforcement.
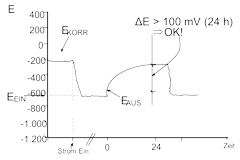
In order to fully assess the quality of the protection achieved by the applied CP-B system, further sensors such as reference electrodes (RE) are mounted adjacent to the reinforced steel. These electrodes record the electro-chemical potential in relation to designates potentials. The most widely used and accepted criterion for verifying the effectiveness of CP system is the 100 mV depolarization (switch-off) criterion. In principal, this confirms the reinforced steel is suitably protected when there is a potential drop of at least 100 mV recorded over a period of 24 hours, after the power source has been switched-off.
It should be noted that the individual reading are specifically related to the correct positioning of the reference electrode, and furthermore that the required quantity and positions of the RE’s are critical in the correct assessment of the structure (> 1 RE per 100 m2).
Anode Type CP-B Systems
Depending on the state of the structure and it's reinforcement and susceptibility to Corrosion, the following Anode type CP-B systems can be used for the structure :
Economic, Environmental & Ecological Advantages :
- Shorter Construction time,
- Repair are localized and can be undertaken during on-going operations,
- Lower Energy Expenses
- Reduction of chloride contaminated concrete,
- Minimized interference/disturbance to the local environment and existing structure.
- Reduced noise pollution and vibration emissions
Highly recommended for Underground & Multi-Storey parking:
- Localized and phased application of the system
- Avoidance of large scale closure and hence loss of income
Improved Traceability & Sustainability:
- Continuous operation of the structure
- Continuous monitoring through built-in sensors
- Regular on-going maintenance programs
BBV Services:
- Preliminary structural Investigation
- Consultation during planning of a remediation system
- Detailed planning and selection of an appropriate CP system
- Installation of the complete CP-B system
- Operation & Maintenance of a complete CP-B System (cost saving can be achieved through correct application of sensors and provision of remote data recorders and transmitters)